Advanced Parkinson's Disease Study
Still having movement difficulties while taking levodopa?
If unpredictable "OFF" time and limited medication choices are holding you back, the ATLANTIS Study offers a path forward. Help us explore a new class of medication designed to address the unique challenges of Parkinson's.
Why the ATLANTIS Study is Your Chance to Take Control of Parkinson's
Living with Parkinson's means navigating unpredictable "OFF" periods – those times when your medication wears off and symptoms like tremors and stiffness return. It's a frustrating reality, especially as the disease progresses and your brain's natural dopamine production declines.
As Parkinson's progresses, the brain's ability to produce and release dopamine becomes inconsistent, leading to fluctuations in symptom control. This can result in "OFF" periods, where medication effects wear off before the next dose, causing Parkinson's symptoms to return. "OFF" time can be disruptive and unpredictable, affecting your daily life, mobility, and overall well-being.
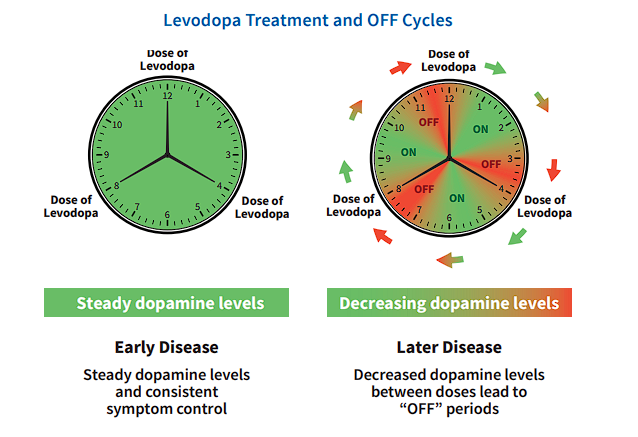
Figure 1. How levodopa levels change over time.
Source:Adapted from ADPA
The ATLANTIS Study offers a path forward.

What We're Doing and Why It Matters
We're testing a new investigational medication called UCB0022 that could potentially reduce the frustrating impact of "OFF" time – those periods when your Parkinson's symptoms return between medication doses. This study will help us understand if UCB0022 is safe, effective, and well-tolerated when taken alongside your current medications.
By participating, you're not just potentially helping yourself; you're contributing to research that could lead to new and better treatments for Parkinson's. Your experience matters.
What To Expect in the ATLANTIS Study
The ATLANTIS Study is a research journey that lasts a little over 4 months and involves 10 visits to our study site. Here's what you can expect during each phase:

- Screening: Our medical team will conduct a thorough assessment, including a review of your medical history, current medications, a physical examination, and some routine tests (like bloodwork and an ECG) to ensure you meet all the study criteria.
- Treatment Period:
- Medication: You'll be randomly assigned to take either one of two doses of UCB0022 (the investigational medication) or a placebo (an inactive substance that looks like the medication) every morning, along with your usual Parkinson's medications.
- Monitoring: You'll keep a diary of your Parkinson's symptoms for three consecutive days on four separate occasions during the study. You'll also complete electronic questionnaires on a tablet twice during the study.
- Movement Tracking (Optional): If you're 46 or older, you may be asked to wear a smartwatch-like device that tracks your movement throughout the day.
- Safety Follow-Up: We'll have one final visit after you've stopped taking the study medication to check on your well-being and gather additional information.

Who can participate in the ATLANTIS study?

To be able to join the ATLANTIS Study, you must:
- Be between 35 and 80 years of age
- Have been diagnosed with Parkinson’s disease 5 or more years ago
- Be experiencing daily motor fluctuations (at least 2 cumulative hours of “OFF” time every day when Parkinson’s disease symptoms return after the medication has worn off)
- Currently be receiving treatment with levodopa (with or without additional therapies for Parkinson’s disease)
- Be willing to complete a 3-day symptom diary at four time points during the study

You cannot participate in the study if you:
- Have had brain surgery for PD (including deep brain stimulation, thalamus surgery, and experimental cell therapy or gene therapy)
- Are using parenteral PD medication (medication that is infused or injected like duopa/duodopa or apomorphine)
- Have a diagnosis of dementia, other forms of important cognitive dysfunction, epilepsy or another seizure disorder
- Have a history or current diagnosis of Type 1 diabetes or uncontrolled Type 2 diabetes or have current untreated hypertension (high blood pressure)
- Have had major depression or psychotic disorder or any other psychiatric condition within the past 5 years
- Have a history of alcohol or drug use disorder
- Have a positive drug/alcohol test, HIV test or hepatitis B test when you join the study
- Other criteria will need to be met in order to participate in this study. Your study team will discuss these with you.
By participating in this study, you will get

Potential access access to Parkinson's study treatment

Close care and monitoring from Parkinson's specialists

The opportunity to help advance the treatment of PD
Your Comfort and Safety Matter:
- The ATLANTIS Study is blinded, which means neither you, your care partner, nor our study team will know whether you're taking UCB0022 or the placebo. This ensures unbiased results.
- We want you to feel fully informed about the study, so we'll provide you with a detailed informed consent document that outlines everything involved.
- We also offer travel support to and from your study visits, as well as reimbursement for reasonable expenses for your participation.
Take the Questionnaire
About SC3 Research Group, Inc.
SC3 Research Group is a clinical research organization dedicated to advancing the understanding and treatment of neurodegenerative disorders like Parkinson's Disease. Our team of expert researchers, clinicians, and support staff work tirelessly to bring innovative therapies and solutions to those living with this challenging condition. By partnering with healthcare providers, biopharmaceutical companies, and other stakeholders, we strive to improve the lives of individuals affected by PD through the power of clinical research.
Frequently Asked Questions (FAQs)
We understand that you may have questions about participating in the ATLANTIS Study. Here are some common questions we hear:
Q. What is a clinical research study
A clinical research study, also called a clinical trial or research study, is a carefully designed scientific evaluation of an study drug. Clinical research studies are conducted by doctors and researchers. A clinical research study helps to answer important questions about an study drug, such as:
- How safe is the study drug?
- Does it work?
All study drugs must be tested in clinical research studies before they can be approved by authorities to be prescribed to patients. Without people taking part in these studies, we would have no new medications.
Our clinical trial, the ATLANTIS Study, is a Phase 2 clinical trial investigating UCB0022, a potential new medication for Parkinson's disease (PD). It aims to assess the medication's effectiveness, safety, tolerability, and how it's processed in the body when taken alongside standard PD treatments.
UCB0022 is a new class of medication that affects how dopamine (a brain chemical) or medications that act like dopamine are made available in the brain. We are testing it to see if it can reduce the time spent in the "OFF" state, when Parkinson's symptoms return between medication doses.
Q. I've read all of the information on this website, and I still have questions about trying a study drug that may not work. Can you give me more information?
There are always risks and benefits of participating in a clinical research study. There is always a chance that the study drug could cause side effects or won’t work. However, you should know that there are strict rules in place to monitor the safety of study participants. Before joining any clinical research study, it is important to consider the risks and understand what they are. The study team will explain all of the risks and benefits at the first study visit. Throughout the study, participants will be closely monitored by a team of local doctors and nurses. They will be there to answer any questions you may have.
Q. Is there a cost to take part?
The study-required investigational study drug or placebo will be provided at no cost. Study-related care from a team of experienced doctors and nurses will also be provided throughout the study at no cost to study participants.
Q. What happens if I or a loved one decide to take part?
If you would like to know whether you or your loved one might be eligible for the study, please fill out the form and will be contacted. If you or your loved one are eligible, we will match you/them to a clinical research study center in your area that is participating in the study. We will also help you schedule your/their first study appointment at the study center. Please note that before being enrolled in the study, additional eligibility criteria will be checked by the study doctor or study team during the screening process.
Q. Will participant confidentiality be protected?
All personal information will remain confidential, and data will only be collected and used as necessary to support an individual’s match to and participation in a study. Participants’ names will not be included in any data reported. For more information, including how and why we process personal data, please read our Privacy Policy.
Q. Are clinical research studies safe?
Like all medications, UCB0022 may have side effects. Your study doctor will discuss all known risks and benefits with you before you decide to participate. Your health and well-being will be closely monitored throughout the study.
Clinical research studies must undergo rigorous reviews and follow strict rules. These rules help to ensure that the rights, safety, and well-being of participants are at the forefront of any study.
Participating in a clinical research study comes with risks as well as benefits. Before joining a study, potential participants must make sure they understand the benefits, such as getting access to study-related medical care and maybe helping others in the future. They must also understand the risks, such as unknown side effects. Potential study participants can take as much time as they need to decide whether to take part and get as much information as they can. Before joining a study, the potential study participant will be asked to sign an informed consent form, which will include a full explanation of the study, including its potential risks.
Q. What else do I need to think about?
It is up to you or your loved one to decide if you/they want to take part in the 202 Parkinson’s Disease Study. Participation in the study is voluntary. Please also consider:
- If you or your loved one decide to participate, you/they can withdraw at any time during the study.
- The study team will explain the possible benefits and risks of the study during the informed consent process.
- You or your loved one do not have to take part in any study if you/they don’t want to.
- A team of doctors and nurses will monitor the health of participants carefully during the study.
- The investigational study drug or placebo will be provided at no cost.
- If you or your loved one decide to participate, you/they may be helping people with Parkinson’s disease in the future.
Participants will need to follow all the instructions from the study doctor and nurses.
Q: Where is the study located?
The ATLANTIS Study is being conducted at SC3 Research in Pasadena, California.
- You can reach us by phone at (626) 250-2070 or visit our website at sc3-research.com for more information or to schedule a consultation.
The ATLANTIS Study is sponsored by UCB Biopharma SRL, a global biopharmaceutical company focused on neurological conditions like Parkinson's.
We encourage you to ask any questions you have. Our study team is here to provide clear and transparent answers.
Are you still looking for potentially more effective ways to manage Parkinson's?
SC3 Research Group in Pasadena is conducting critical research on cutting-edge therapies, and we urgently need participants.
Current medication options can offer relief, but new treatments show promise of significantly improving symptom control and slowing disease progression. Imagine accessing these advancements before they're widely available.
Time is of the essence. Contact us today to discuss potential participation and screening options.
.jpg)
The Atlantis Parkinson’s Disease Study
NOW ENROLLING
Pasadena CA
SC3 Reseach Group, Inc.

Research has changed the way we diagnose, treat, and understand Parkinson's disease and it's made only possible because of people with Parkinson's who participated in clinical trials. Join us in learning better about the condition while participating in studies that will shape the future of Parkinson's Disease and the thousands of people affected by it.
